CH 222 Chemical of the Week
Radiation
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

ENVIRONMENTAL RADIATION
We are exposed to nuclear radiation every day of our lives. Some of this radiation is from natural sources, and some results from human activity. Natural sources include cosmic radiation from space, radiation from lighter, unstable nuclei produced by the bombardment of the atmosphere by cosmic radiation, and radiation from heavy, unstable nuclei produced by the decay of a few long-lived nuclides in the earth's crust. Artificial sources include medical procedures, commercial products that contain radioactive materials, and fallout from nuclear testing.
Nuclear radiation can cause biological damage because it is highly energetic. In passing through matter, nuclear radiation loses its energy by causing ionization in the absorbing material. For this reason, nuclear radiation is called ionizing radiation. In the ionization process, neutral atoms in the absorbing material lose electrons, forming positive ions. Frequently, the ejected electrons possesses sufficient energy to cause ionizations in other atoms. The average amount of energy required to ionize an atom is about 35 electron volts. (An electron volt is the amount of energy acquired by an electron accelerated in an electric field of 1 volt. It is equivalent to 1.6 × 10–19 Joule.) The energy of a single particle from a nuclear decay can be as high as 8 million electron volts (8 MeV). This energy is dissipated by producing ions, and an 8-MeV particle can produce 2 × 105 ions.
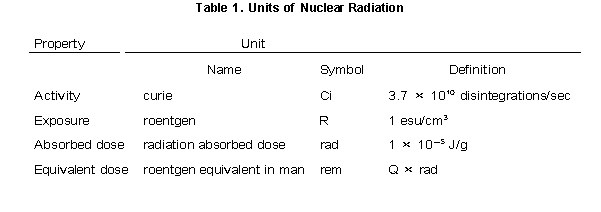
The magnitude of radioactivity in a sample of radioactive material is expressed using several different units (Table 1). One type of unit focuses on the number of decaying nuclei and is called the activity. Activity is expressed in terms of disintegrations per time. The most common unit of activity is the curie (abbreviated as Ci). It is defined as 3.7 × 1010 disintegrations per second. This happens to be the activity of 1 gram of Ra-226, which was discovered by Marie Curie. The SI unit of activity is the becquerel, which is 1 disintegration per second. The other units of radioactivity focus on the effects of radiation on the surroundings. The exposure expresses the amount of ionization caused by radioactive material. The common unit of exposure is the roentgen, which is defined as the amount of radiation that produces, in 1 cm3 of dry air, ions having a total charge of 1 electrostatic unit. In SI units, the roentgen is equivalent to 2.58 × 104 Coulomb/kg of air. The absorbed dose of radioactivity expresses the amount of energy absorbed by a substance exposed to ionizing radiation. One such absorbed dosage unit is the radiation absorbed dose, rad, which is 1 × 105 Joule/g. Different kinds of radiation will cause different biological effects for the same amount of energy absorbed. For this reason, the unit called the roentgen equivalent in man, or rem, was introduced. The rem is equal to the rad multiplied by a factor, Q, which accounts for the relative biological effect of radiation on humans. For any radiation (and for X-rays) Q ~ 1, while for particles and fast neutrons, Q ~ 20.
The ionizing power of radiation depends on the type of radiation. An alpha particle, which is relatively massive, is quite efficient at producing ions, ionizing virtually every atom in its path. Alpha particles lose most of their energy after traveling only a few centimeters in air or less than 0.005 mm in aluminum. A beta particle, which is relatively light, ionizes only a fraction of the atoms in its path. Beta particles travel more than a meter in air or several millimeters in aluminum.
For most humans, much of the absorbed radiation is cosmic radiation. At sea level, the average human absorbs about 26 millirem (mrem) per year. The atmosphere shields the surface of the earth from cosmic radiation, but for each 100-meter increase in elevation, the dosage absorbed increases by about 1.5 mrem per year. A person traveling by commercial jet aircraft can receive as much as 10 mrem on a long flight, such as Los Angeles to London.
When cosmic radiation interacts with the gases in the atmosphere, it causes nuclear transformations that release particles such as neutrons and protons. These neutrons and protons interact with other nuclei in the atmosphere, producing radioactive nuclei, such as carbon-14 and tritium (hydrogen-3). Carbon-14 is responsible for less than 1 mrem per year of absorbed radiation in humans, and tritium only about 1 microrem.
Long-lived radioisotopes in the earth's crust are also a source of absorbed radiation. One of these that is particularly significant is potassium-40, with a half life of 1.3 × 109 years and making up only 0.019% of all potassium. It is significant because potassium is one of the most abundant elements and because it is an essential component of foods. The average absorbed dose for humans from external potassium-40 is about 12 mrem per year, while that from internal potassium-40 is about 20 mrem per year.
For more information about environmental radiation, see "Radioactivity in Everyday Life," an article in the May, 1997 issue of the Journal for Chemical Education (page 501).
URANIUM: A RADIOACTIVE CLOCK
How old is the Earth, the solar system, or a piece of charcoal from an ancient campfire? Until the beginning of the 20th Century, geologists had no method by which to determine the absolute age of a material. The age of the earth was believed to be at most tens of millions of years. Not long after the discovery of radioactivity in 1896, scientists realized that radioactive decay constitutes a "clock" capable of measuring absolute geologic time. By 1907, the discovery that lead was the final product of uranium decay provided evidence that geologic age needed to be reckoned not in millions, but in billions of years.
Uranium occurs in numerous minerals, such as pitchblende (UO3·UO2·PbO) and carnotite (K2O·2U2O3·V2O5·3H2O). It is not all that rare, being more plentiful in the Earth's crust than mercury and silver. The metal was first isolated in 1841 by the reduction of uranium(IV) chloride with potassium.
4 K + UCl4 ![]() 4 KCl + U
4 KCl + U
Uranium is sufficiently radioactive to expose a photographic plate in about an hour. Naturally occurring U contains 14 isotopes, all of which are radioactive. The three most abundant are U-238 (99.28%), U-235 (0.71%), and U-234 (0.006%). In contrast to chemical reactions, where the isotopes of an element behave similarly, in nuclear reactions isotopes behave quite differently. This reveals itself in the different half lives of these isotopes, and in the fact that among these three only U-235 undergoes fission.
The most abundant of the naturally occurring uranium isotopes decays by alpha emission to Th-234.
238U ![]() 234Th + 4He (t½ = 4.5 × 109 years)
234Th + 4He (t½ = 4.5 × 109 years)
The product of this reaction, Th-234, is also radioactive and undergoes beta decay.
234Th ![]() 234Pa + -1e (t½ = 24 days)
234Pa + -1e (t½ = 24 days)
Protactinium-234 also decays by emitting a beta particle. These are only the beginning of a series of 14 nuclear decay steps. After emission of eight alpha particles and six beta particles, the isotope Pb-206 is produced. It is a stable isotope that does not disintegrate further. The complete process is called the uranium radioactive decay series. The intermediate isotopes are called "daughters." The half lives of the daughters range from 1.6 × 10-14 seconds for Po-214 to 2.5 × 105 years for U-234. Two other such radioactive series occur in nature. They start with U-235 and Th-232.
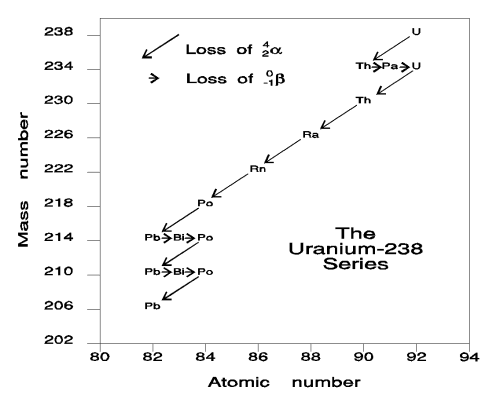
The uranium radioactive series has been used to estimate the age of the oldest rocks in the Earth's crust. The ratio of U-238 to Pb-206 in a rock changes slowly as the U-238 in the rock decays. Because the half life of U-238 is 20,000 times that of the next longest half life in the series, the rate of decay of U-238 is the rate-determining step in the conversion of U-238 to Pb-206. The rate of radioactive decay is first order in the amount of decaying isotope. Therefore, the first order equation relates the amount of isotope in a sample to time.
![]()
In this equation, N0 is the number of U-238 atoms initially present in the sample, N is the number of U-238 atoms in the sample after a length of time t has elapsed, and lambda is the decay constant of U-238. If no Pb-206 was initially present in the sample, then N0 is equal to the sum of the number of atoms of U-238 and Pb-206.
At least two other radioactive clocks are used for dating geological time spans. These are the potassium to argon and rubidium to strontium transformations. Potassium-40 decays by electron capture to argon-40.
40K + -1e ![]() 40Ar (t½ = 1.3 × 109 years)
40Ar (t½ = 1.3 × 109 years)
In the rubidium-strontium transformation, Rb-87 emits a beta particle to form Sr-87.
87Rb ![]() 87Sr + -1e (t½ = 5.7 × 1010 years)
87Sr + -1e (t½ = 5.7 × 1010 years)
These radioactive clocks are more useful for dating rock samples than the uranium clock, because both potassium and rubidium are more widely distributed in rock samples than is uranium.
All radiochemical methods of dating have some uncertainties associated with them. Several assumptions must be made in determining an age. Perhaps the most significant assumption is the supposition that the sample was a closed system throughout its existence, that is, no parent or daughter isotope was gained or lost. Another assumption involves the amount of daughter isotope present at the formation of the sample. Generally, this is taken as zero for rare isotopes. The strongest evidence for the age of a sample is obtained when two different radiochemical dating methods produce the same result. Because the chemical properties of daughter products are so very different, any geological transformation of a rock sample will have quite different effects on the sample's daughter isotope contents. Potassium and rubidium frequently occur together in rock samples, making this pair particularly important for radiochemical dating.
Radiochemical dating of samples from the Earth's crust yield a maximum age of about 3.5 × 109 years. The Earth is believed to be older than this. The oldest meteorites and moon rocks are 4.5 × 109 years old. If these other members of the solar system were formed at the same time, then perhaps the Earth itself was formed 4.5 billion years ago. The isotopic composition of lead supports this conclusion. Of the four lead isotopes, only Pb-204 is not produced by radioactive decay of parent U-238, U-235, or Th-232. Comparing the isotopic composition of lead in the Earth's crust to that of meteorites free of uranium and thorium indicates that about 4.5 billion years of U and Th decay would be required to produce the Pb isotope ratios found on Earth.
EXERCISE: Write a (less than 500 words) summary of nuclear fusion and nuclear fission - do you support them, are you against them, etc. Include at least three internet or library references to support your position.
Questions? Contact me!