CH 222 Chemical of the Week
Gases of the Air
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

GASES OF THE AIR
The air around us is a mixture of gases, mainly nitrogen and oxygen, but containing much smaller amounts of water vapor, argon, and carbon dioxide, and very small amounts of other gases. Air also contains suspended dust, spores, and bacteria. Because of the action of wind, the percent composition of air varies only slightly with altitude and location. The table indicates the composition of a typical sample of air after all water vapor and suspended particles have been removed.
 The amount of water in the air varies tremendously with location, temperature, and time. In deserts and at low temperatures, the content of water vapor can be less than 0.1% by volume. In warm, humid zones, the air may contain over 6% water vapor.
The amount of water in the air varies tremendously with location, temperature, and time. In deserts and at low temperatures, the content of water vapor can be less than 0.1% by volume. In warm, humid zones, the air may contain over 6% water vapor.
Air is the commercial source for many of the gases it contains. It is separated into its components by fractional distillation of liquefied air. Before air is liquefied, water vapor and carbon dioxide are removed, because these substances solidify when cooled and would clog the pipes of the air liquefaction plant. The dry, CO2-free air is compressed to about 200 atmospheres. This compression causes the air to become warm, and the heat is removed by passing the compressed air through radiators. The cooled, compressed air is then allowed to expand rapidly. The rapid expansion causes the air to become cold, so cold that some of it condenses. By the alternate compressing and expanding of air, most of it can be liquefied.
Nitrogen is obtained from liquid air by distillation at -196°C. The gas obtained by this process is actually a mixture of nitrogen and about 1.25% noble (or "inert") gases, argon, neon, krypton, and xenon. Nitrogen is second only to sulfuric acid in the volume produced by the U.S. chemical industry. Its major uses are as an inert blanketing atmosphere in chemical processing (14%), electronics (15%), and, in liquid form, as a freezing agent (21%). Nitrogen is used to make agricultural fertilizers, such as ammonia and nitrates. It is also used in the production of acrylonitrile, CH2=CHCN, which is important in the manufacture of synthetic fibers such as Orlon, and in the production of cyanamide, HN=C=NH, which is polymerized to Melamine plastic. Because it is a very poor oxidizing agent, nitrogen is used to pack oxidizable foods, such as ground coffee, and as an inert atmosphere in the manufacture of electronic components. Liquefied nitrogen, because it is very cold, is used extensively to chill materials for preservation, as in freeze-drying of foods, and in manufacturing processes that require low temperatures, such as machining of aluminum.
The lighter noble gas neon is obtained from air. Its boiling point (-246°C) is too low for neon to condense during the liquefaction of air, and neon concentrates in the gas that remains after air is liquefied. (This remaining gas also contains helium, but the major commercial source of helium is natural gas, in which its concentration is much higher than it is in air.) The heavier noble gases argon, krypton, and xenon are obtained by the fractional distillation of liquid air. Under the proper conditions, a fraction containing roughly 60% noble gases, 30% oxygen, and 10% nitrogen can be obtained from liquid air. Oxygen is removed from the mixture by passing it over hot copper. Oxygen reacts with hot copper to form copper(II) oxide, CuO. The remaining gas is a mixture of noble gases and nitrogen. This mixture is the gas used to fill incandescent light bulbs. Nitrogen is removed from the mixture by passing the mixture over hot magnesium, which reacts with nitrogen to form magnesium nitride, Mg3N2. The remaining gas is a mixture of argon, neon, krypton, and xenon. Because these elements are chemically very unreactive, chemical means cannot be used to separate them. They are separated by adsorbing the liquid mixture onto activated charcoal at very low temperature. As the activated charcoal is warmed slowly, each gas desorbs individually in a particular temperature range. When the temperature is raised to -80°C, the gas that escapes is nearly pure argon. As the temperature is raised to higher temperatures, nearly pure krypton, and then xenon, are released.
Argon is the most abundant and most used of the noble gases. Its chief uses are in metallurgy, where it provides an inert atmosphere in which hot metals can be worked. Because argon is so very unreactive, it prevents chemical reactions of the very hot metal being welded or forged. Perhaps the most familiar use of the noble gases is in "neon" signs. These are lamps constructed usually from clear, colorless glass tubes filled with a gas which emits light when an electric current passes through it. Pure neon in such a tube produces orange-red light, argon produces blue-green. (Other gases are also used; for example, mercury vapor produces blue light. Other colors are produced by using colored tubes, mixtures of gases, and fluorescent coatings inside the tubes.)
Nearly all commercial oxygen (over 95%) is produced by fractional distillation of liquid air. It boils at -183°C. Oxygen is the third highest-volume chemical produced in the U.S., and most of this product is more than 99.5% pure. Oxygen is paramagnetic, that is, it is attracted to a magnet. Liquid oxygen is pale blue. The major commercial uses of oxygen are in metal manufacturing (30%), metal fabricating (33%), and in health services (13%). In the steel industry, oxygen is passed through impure molten iron in a blast furnace to oxidize and remove impurities such as carbon, sulfur, phosphorus, and silicon. Oxygen is also used as the oxidant in torch cutting of steel. In this process, the steel is heated by an oxygen-acetylene flame, and a stream of hot oxygen is directed at the hot steel. The hot steel is oxidized by the hot oxygen and erodes away, severing the steel. Oxygen is also used extensively in the chemical industry, such as in the production of nitric acid, HNO3, from ammonia, NH3.
The reaction of oxygen with another gas can lead to an explosion. There are two reasons why a reaction results in an explosion, and therefore, two types of explosions. Thermal explosions result from temperature effects on the rate of a reaction. Chain reaction explosions result from the behavior of the reacting molecules.
In a thermal explosion, the heat released by the reaction increases the temperature of the reacting mixture. This increased temperature increases the rate of the reaction. If heat released by the reaction cannot escape, the rate of the reaction increases rapidly, and an explosion can result. Heat is likely to build up in a reacting gas mixture when the pressure is high, because under these conditions, individual "hot" molecules cannot move very far and the energy is contained. Thus, thermal explosions are likely to occur at high pressures.
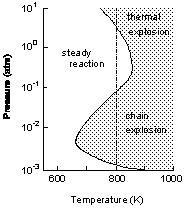 In a chain reaction explosion, the reaction occurs in such a way that the number of highly reactive particles (called free radicals) increases during the reaction. The reaction of hydrogen and oxygen occurs in such a fashion. If the pressure is very low, free radicals are likely to collide with the walls of the container before reacting with other molecules, and no explosion occurs. If the pressure is high, the free radicals are likely to collide with each other, terminating a reaction chain, and no explosion occurs. Only when free radicals collide with molecules and generate other free radicals does the chain reaction continue, producing an explosion. Thus, chain reaction explosions are likely to occur at intermediate pressures.
In a chain reaction explosion, the reaction occurs in such a way that the number of highly reactive particles (called free radicals) increases during the reaction. The reaction of hydrogen and oxygen occurs in such a fashion. If the pressure is very low, free radicals are likely to collide with the walls of the container before reacting with other molecules, and no explosion occurs. If the pressure is high, the free radicals are likely to collide with each other, terminating a reaction chain, and no explosion occurs. Only when free radicals collide with molecules and generate other free radicals does the chain reaction continue, producing an explosion. Thus, chain reaction explosions are likely to occur at intermediate pressures.
The figure shows how the explosiveness of a hydrogen-oxygen mixture depends on temperature and pressure. Explosion occurs in the shaded area. At 800K and low pressure, no explosion occurs. As the pressure is raised, the mixture enters the chain reaction explosion region. As the pressure is raised further, the mixture is no longer explosive. When the pressure is increased even more, the mixture enters the thermal explosion region.
EXERCISE: What is the approximate average molar mass of air? Show calculations and set up, and give citation (source) used to find your information.
Questions? Contact me!