CH 222 Chemical of the Week
Carbon Dioxide
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

CARBON DIOXIDE
Carbon dioxide, CO2, is one of the gases in our atmosphere, being uniformly distributed over the earth's surface at a concentration of about 0.033% or 330 ppm. Commercially, CO2 finds uses as a refrigerant (dry ice is solid CO2), in beverage carbonation, and in fire extinguishers. In the United States, 10.89 billion pounds of carbon dioxide were produced by the chemical industry in 1995, ranking it 22nd on the list of top chemicals produced. Because the concentration of carbon dioxide in the atmosphere is low, it is not practical to obtain the gas by extracting it from air. Most commercial carbon dioxide is recovered as a by-product of other processes, such as the production of ethanol by fermentation and the manufacture of ammonia. Some CO2 is obtained from the combustion of coke or other carbon-containing fuels:
C(coke) + O2(g) ![]() CO2(g)
CO2(g)
Carbon dioxide is released into our atmosphere when carbon-containing fossil fuels such as oil, natural gas, and coal are burned in air. As a result of the tremendous world-wide consumption of such fossil fuels, the amount of CO2 in the atmosphere has increased over the past century, now rising at a rate of about 1 ppm per year. Major changes in global climate could result from a continued increase in CO2 concentration.
In addition to being a component of the atmosphere, carbon dioxide also dissolves in the water of the oceans. At room temperature, the solubility of carbon dioxide is about 90 cm3 of CO2 per 100 mL of water. In aqueous solution, carbon dioxide exists in many forms. First, it simply dissolves.
CO2(g) ![]() CO2(aq)
CO2(aq)
Then, an equilibrium is established between the dissolved CO2 and H2CO3, carbonic acid.
CO2(aq) + H2O(l) ![]() H2CO3(aq)
H2CO3(aq)
Only about 1% of the dissolved CO2 exists as H2CO3. Carbonic acid is a weak acid which dissociates in two steps.
| H2CO3 | H+ + HCO3-1 | Ka1 = 4.2 x 10-7 | |
| HCO3-1 | H+ + CO32- | Ka2 = 4.8 x 10-11 |
As carbon dioxide dissolves in sea water, an equilibrium is established involving the carbonate ion, CO32-. The carbonate anion interacts with cations in seawater. According to the solubility rules, "all carbonates are insoluble except those of ammonium and Group IA elements." Therefore, the carbonate ions cause the precipitation of certain ions. For example, Ca2+ and Mg2+ ions precipitate from large bodies of water as carbonates. For CaCO3, the value of Ksp is 5 x 10-9, and for MgCO3, Ksp is 2 x 10-3. Extensive deposits of limestone (CaCO3) and dolomite (mixed CaCO3 and MgCO3) have been formed in this way. Calcium carbonate is also the main constituent of marble, chalk, pearls, coral reefs, and clam shells.
Although "insoluble" in water, calcium carbonate dissolves in acidic solutions. The carbonate ion behaves as a Bronsted base.
CaCO3(s) + 2 H+(aq) ![]() Ca2+(aq) + H2CO3(aq)
Ca2+(aq) + H2CO3(aq)
The aqueous carbonic acid dissociates, producing carbon dioxide gas.
H2CO3(aq) ![]() H2O(l) + CO2(g)
H2O(l) + CO2(g)
In nature, surface water often becomes acidic because atmospheric CO2 dissolves in it. This acidic water can dissolve limestone.
CO2(aq) + H2O(l) + CaCO3(s)
![]() Ca2+(aq) + 2 HCO3 -1(aq)
Ca2+(aq) + 2 HCO3 -1(aq)
This reaction occurs in three steps.
| CaCO3(s) | Ca2+(aq) + CO32-(aq) | |
| CO2(aq) + H2O(l) | H2CO3(aq) | |
| H2CO3(aq) + CO32- (aq) | 2 HCO3-1(aq) |
In the third step, carbonate ions accept hydrogen ions from carbonic acid. This reaction often occurs underground, when rainwater saturated with CO2 seeps through a layer of limestone. As the water dissolves calcium carbonate, it forms openings in the limestone. Caves from which the limestone has been dissolved are often prevalent in areas where there are large deposits of CaCO3 (e.g., Mammoth Cave, Carlsbad Caverns, and Cave of the Mounds). If the water containing dissolved Ca(HCO3)2 reaches the ceiling of a cavern, the water will evaporate. As it evaporates, carbon dioxide escapes, and calcium carbonate deposits on the ceiling.
Ca2+(aq) + 2 HCO3(aq) ![]() H2O(g) + CO2(g)
+ CaCO3(s)
H2O(g) + CO2(g)
+ CaCO3(s)
Similar chemical reactions are also responsible for the erosion of marble and limestone monuments of historical and cultural importance, such as the Taj Mahal in India, the Mayan temples in Mexico and Guatemala, and the Rock Churches of Ethiopia. Here, the acid involved is likely to be H2SO3 or H2SO4, formed when the atmospheric pollutants SO2 and SO3 dissolve in rain water. The carbonate stone is damaged by the conversion of the relatively insoluble carbonate to the more soluble sulfate.
CaCO3(s) + H2SO4(aq)(in rain) ![]() CaSO4(s)
+ H2O(l) + CO2(g)
CaSO4(s)
+ H2O(l) + CO2(g)
The calcium sulfate is eroded away as it slowly dissolves in rain water (the Ksp of CaSO4 is 3 x 10-5). The life of these monuments is now being extended by treating them with a mixture of barium hydroxide, Ba(OH)2, and urea, (NH2)2CO. This mixture soaks into the porous marble or limestone. Gradually, the urea decomposes to ammonia and carbon dioxide.
(NH2)2CO(aq) + H2O(l)
![]() 2 NH3(aq) + CO2(aq)
2 NH3(aq) + CO2(aq)
This carbon dioxide combines with the barium hydroxide to form barium carbonate.
CO2(aq) + Ba(OH)2(aq) ![]() BaCO3(s) + H2O(l)
BaCO3(s) + H2O(l)
Barium carbonate is more resistant to erosion because it is less soluble than calcium carbonate. For BaCO3 the Ksp is 2 x 10-9. Furthermore, barium sulfate is even less soluble, with a Ksp of 1 x 10-10. When barium carbonate on the surface of the treated monument reacts with sulfur dioxide in the air, it forms a layer of barium sulfate which protects the monument.
2 BaCO3(s) + 2 SO2(g) + O2(g)
![]() 2 BaSO4(s) + 2 CO2(g)
2 BaSO4(s) + 2 CO2(g)
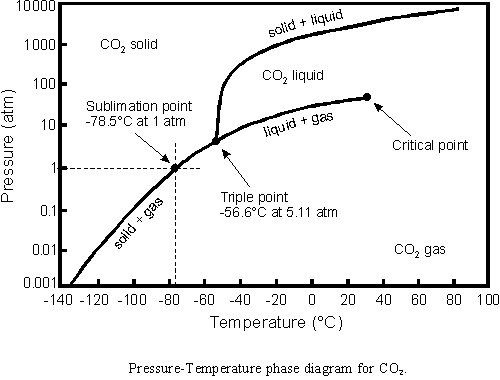
A new use for liquid carbon dioxide currently under development is as a dry-cleaning solvent. Currently, most laundries use chlorinated hydrocarbons as dry-cleaning solvents. These chlorinated hydrocarbons are probable human carcinogens, so the search is on for replacements. Carbon dioxide does not exist in liquid form at atmospheric pressure at any temperature. The pressure-temperature phase diagram of CO2 shows that liquid carbon dioxide at 20 °C requires a pressure of 30 atmospheres. The lowest pressure at which liquid CO2 exists is at the triple point, namely 5.11 atm at -56.6 °C. The high pressures needed for liquid CO2 require specialized washing machines. Like chlorinated hydrocarbons, liquid carbon dioxide is an effective solvent for grease and oils. Liquid CO2 has some advantages over chlorinated hydrocarbons--items that cannot be dry cleaned with chlorinated hydrocarbons, such as leather, fur, and some synthetics, can be safely cleaned with liquid carbon dioxide. More information about alternative dry-cleaning solvents can be found in the Innovations section of Environmental Health Perspectives, Volume 104, Number 5.
EXERCISE: What phase of CO2 exists at 0.065 atm and -110 degrees Celsius?
Questions? Contact me!