CH 222 Chemical of the Week
Aspirin
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

ASPIRIN, C9H8O4
One of the best known aromatic acetates is acetylsalicylic acid, or aspirin, which is prepared by the esterification of the phenolic hydroxyl group of salicylic acid.
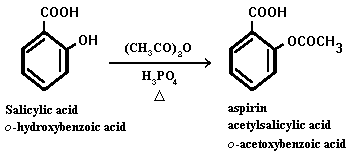
Aspirin possesses a number of properties that make it the most often recommended drug. It is an analgesic, effective in pain relief. It is also an anti-inflammatory agent, providing some relief from the swelling associated with arthritis and minor injuries. Aspirin is also an antipyretic compound, which means it reduces fever. Each year, more than 40 million lb of aspirin is produced in the US alone, a rate that translates to about 300 tablets per year for every man, woman and child. However, it is not so innocuous a drug as one might imagine from its widespread use and ready availability. Repeated use may cause gastrointestinal bleeding, and large doses can provoke a host of reactions including vomiting, diarrhea, vertigo and hallucinations. The a verage dose is 0.3-1 g; single doses of 10-30 g can be fatal.
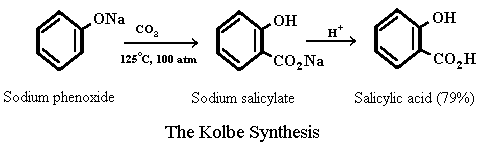
The key compound in the synthesis of aspirin, salicylic acid, is prepared from phenol by a process discovered over 100 years ago by the German chemist Hermann Kolbe. In the Kolbe synthesis (also known as the Kolbe-Schmitt reaction) sodium p henoxide is heated with CO2 under pressure and the reaction mixture is subsequently acidified to yield salicylic acid.
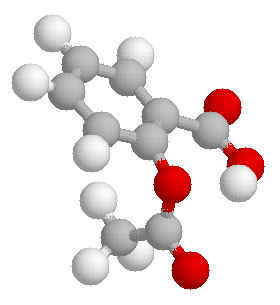
The following image is a three dimensional representation of aspirin.
References:
- Introduction to Organic Chemistry, Streitweiser and Heathcock, MacMillan, NY (1981).
- Organic Chemistry, F.A. Carey, McGraw-Hill, NY, (1987).
EXERCISE: Write a short history of the discovery and development of aspirin. Include any references used for the report.
Questions? Contact me!