CH 222 Chemical of the Week
Agricultural Fertiizers
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

AGRICULTURAL FERTILIZERS:
NITROGEN, POTASSIUM, AND PHOSPHORUS
Anyone who has grown a garden, maintained a lawn, or kept house plants knows that it is necessary to apply a fertilizer to the soil to keep cultivated plants healthy. As they grow, plants extract nutrients they need from the soil. Unless these nutrients are replenished, plants will eventually cease to grow. In nature, nutrients are returned to the soil when plants die and decay. However, this does not occur with cultivated plants. Humans cultivate plants mainly for food, either for themselves or for livestock. When cultivated plants are harvested, the nutrients that the plants extracted from the soil are taken away. To keep the soil productive, it is necessary to replace these nutrients artificially. The kinds and amounts of nutrients that plants need have been determined and can be supplied by applying to the soil substances that contain these nutrients.
A plant contains a great number of chemical compounds. The major compound in all plants is water. The percent of the plant's weight that is water varies greatly from one kind of plant to another, from less than 20% to more than 90%. After the water is removed, the bulk of the dry plant material is carbohydrate, compounds containing the elements carbon, hydrogen, and oxygen. Using the energy of sunlight in a process called photosynthesis, plants make carbohydrates in their leaves. The carbon and oxygen in carbohydrates comes from carbon dioxide, which the plant absorbs from the air, and the hydrogen comes from water absorbed both through the roots and through the leaves. About 90% of the weight of carbohydrates is carbon and oxygen. Therefore, a plant obtains around 90% of its dry weight from the air.
Although carbohydrates account for most of the dry weight of a plant, the plant contains smaller amounts of other compounds that are necessary for its growth. Plants contain proteins, which are essential in the chemical reactions of photosynthesis. Proteins contain the element nitrogen in addition to carbon, oxygen, and hydrogen. Some proteins contain sulfur as well. Plants also contain DNA, which carries the genetic information that controls how a plant grows. DNA contains the element phosphorus, in addition to nitrogen, carbon, oxygen, and hydrogen. Phosphorus is also significant in the storage and distribution of energy throughout the plant. Chlorophyll, the compound that makes plant leaves green and is central in photosynthesis, also contains the element magnesium. The fluids inside the plant's cells also contain other dissolved minerals, which provide the proper environment for the many chemical reactions that occur in the fluid. Among these minerals are compounds of potassium and calcium.

Plants must obtain the elements essential for their growth, other than carbon, oxygen, and hydrogen, from the soil. Thirteen elements essential for plant growth have been identified, and these are listed in the table on the previous page. These essential elements are called nutrients; those needed in the greatest amount are called macronutrients, those needed in lesser amounts are called micronutrients.
Among the macronutrients are nitrogen, phosphorus, and potassium. These three elements are those most rapidly removed from the soil by plants. Therefore, many commercial plant fertilizers supply these three essential elements.
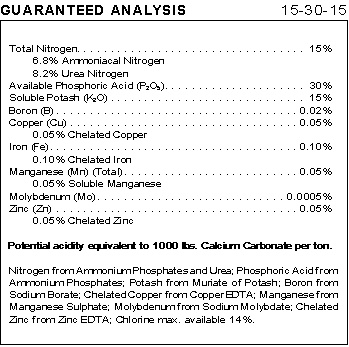 The amount of each element is indicated by N-P-K numbers. The "guaranteed analysis" table (taken from a package of garden fertilizer) shows an N-P-K rating of 15-30-15. These numbers indicate the percent by weight of nitrogen, phosphorus oxide, and potassium oxide in the fertilizer. The 15-30-15 rating indicates that 15% by weight of the fertilizer is nitrogen (N). It also indicates that the weight of phosphorus in the fertilizer is the same as it would be if the fertilizer contained 30% phosphorus oxide (P2O5). The amount of potassium in the fertilizer is the same as it would be if the fertilizer were 15% potassium oxide (K2O).
The amount of each element is indicated by N-P-K numbers. The "guaranteed analysis" table (taken from a package of garden fertilizer) shows an N-P-K rating of 15-30-15. These numbers indicate the percent by weight of nitrogen, phosphorus oxide, and potassium oxide in the fertilizer. The 15-30-15 rating indicates that 15% by weight of the fertilizer is nitrogen (N). It also indicates that the weight of phosphorus in the fertilizer is the same as it would be if the fertilizer contained 30% phosphorus oxide (P2O5). The amount of potassium in the fertilizer is the same as it would be if the fertilizer were 15% potassium oxide (K2O).
The sources of nitrogen used in fertilizers are many, including ammonia (NH3), diammonium phosphate ((NH4)2HPO4), ammonium nitrate (NH4NO3), ammonium sulfate ((NH4)2SO4), calcium cyanamide (CaCN2), calcium nitrate (Ca(NO3)2), sodium nitrate (NaNO3), and urea (N2H4CO). Phosphorus is generally supplied as a phosphate, such as diammonium phosphate ((NH4)2HPO4) or calcium dihydrogen phosphate (Ca(H2PO4)2). Potassium comes from potassium sulfate (K2SO4) or potassium chloride (KCl), which is also called muriate of potash.
The phosphorus content of a fertilizer is specified as the amount of P2O5, because this is the anhydrous form of phosphoric acid. In this sense it is the most concentrated form of phosphate, which is the form of phosphorus required by plants. The potassium content is designated in terms of K2O, which is also called potash. Potash is a component of the residue left when plant materials are incinerated. The spreading of ashes on fields is an ancient method of replenishing potassium. Modern chemical fertilizers usually contain KCl instead, but the potassium content is still specified as the equivalent amount of potash. Potassium chloride is 52% by weight K. Potash is 83% potassium. Thus, KCl provides only about 2/3 as much potassium as the same weight of K2O. Thus, if a fertilizer is 25% KCl by weight, its potassium rating, based on potash, would be only 16.
EXERCISES: Draw Lewis structures for these agricultural fertilizers: ammonium nitrate and calcium cyanamide. What are the geometries of the ammonium ion, the nitrate ion, and the cyanamide ion? Please note: You must submit this Chemical of the Week with a true picture or graphic of the Lewis structure, so email your answer to the instructor directly (or give a hard copy to the instructor during lecture, etc.)
Questions? Contact me!