CH 222 Chemical of the Week
LSD
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

Lysergic Acid Diethylamide - LSD, C20H25N3O
 LSD is one of the most powerful hallucinogenic
drugs known. It was invented in 1938 by a Swiss chemist named Albert Hoffman, who
was interested in developing medicines from compounds in ergot, a fungus that attacks
rye. Although LSD is purely synthetic, clues to its biological activity can be found
by tracing the history of the fungus it is derived from.
LSD is one of the most powerful hallucinogenic
drugs known. It was invented in 1938 by a Swiss chemist named Albert Hoffman, who
was interested in developing medicines from compounds in ergot, a fungus that attacks
rye. Although LSD is purely synthetic, clues to its biological activity can be found
by tracing the history of the fungus it is derived from.
Ergot
Ergot of rye is produced by a lower fungus (Claviceps purpurea) that grows parasitically on rye and, to a lesser extent, on other species of grain and on wild grasses. Kernels infested with this fungus develop into light-brown to violet-brown curved pegs (sclerotia) that push forth from the husk in place of normal grains. Ergot of rye (Secale cornutum) is the variety used medicinally.
Ergot has a fascinating history. Once it was dreaded as a poison, but over the course of time it has become to be regarded as a rich storehouse of valuable medicines. Ergot was first mentioned in the early Middle Ages, as the cause of outbreaks of mass poisonings affecting thousands of persons at a time. The illness appeared in two characteristic forms, one gangrenous (ergotismus gangraenosus) and the other convulsive (ergotismus convulsivus). Popular names for ergotism - such as "mal des ardents", "ignis sacer", "heiliges Feuer" or "St. Anthony's fire" - refer to the gangrenous form of the disease. The patron saint of ergotism victims was St. Anthony, and it was primarily the Order of St. Anthony that treated these patients. Until quite recently, outbreaks of ergot poisoning approaching epidemic proportions were recorded in most European countries including certain areas of Russia. However, in the seventeenth century it was discovered that ergot-containing bread was the cause of the poisonings. This, coupled with progress in agriculture, caused the frequency and extent of ergotism epidemics to diminish considerably. The last great epidemic occurred in certain areas of southern Russia in the years 1926-27.
Ergot as a Medicine
The first mention of a medicinal use of ergot, as a drug to precipitate childbirth, is found in the notes of the Frankfurt city physician Adam Lonitzer in 1582. Although ergot had been used since olden times by midwives, it was not until 1808 that this drug gained entry into academic medicine. The use of ergot for these purposes did not last, however, since the uncertainty of dosage led to uterine spasms and dangers to the child.
The early 1930s brought a new era in ergot research, beginning with the determination of the chemical structure of the main chemically active agents, the ergot alkaloids. Finally, W. A. Jacobs and L.C. Craig of the Rockefeller Institute of New York succeeded in isolating and characterizing the nucleus common to all ergot alkaloids. They named it lysergic acid.
The Discovery of LSD
 In the late 1930s, Albert Hoffman was working in the pharmacological
department of Sandoz, in Basel, Switzerland. He was studying derivatives of lysergic
acid, including systematically reacting the acid group with various reagents, to
produce the corresponding amides, anhydrides, esters, etc. One of these derivatives
was the diethylamide, made by addition of the -N(C2H5)2 group, and it was named LSD-25. But the new substance didn't appear to have any particularly useful medical properties, although the research report noted, in passing, that "the experimental animals became restless during the narcosis". For the next five years, nothing more was heard of the substance LSD-25.
In the late 1930s, Albert Hoffman was working in the pharmacological
department of Sandoz, in Basel, Switzerland. He was studying derivatives of lysergic
acid, including systematically reacting the acid group with various reagents, to
produce the corresponding amides, anhydrides, esters, etc. One of these derivatives
was the diethylamide, made by addition of the -N(C2H5)2 group, and it was named LSD-25. But the new substance didn't appear to have any particularly useful medical properties, although the research report noted, in passing, that "the experimental animals became restless during the narcosis". For the next five years, nothing more was heard of the substance LSD-25.
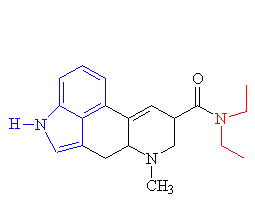 |
| The structure of lysergic acid diethylamide. The diethylamide group is shown in red and the indole ring in blue. |
But for some reason, Hoffman could not forget the relatively uninteresting LSD-25. In his book he says:
"A peculiar presentiment - the feeling that this substance could possess properties other than those established in the first investigations - induced me, five years after the first synthesis, to produce LSD-25 once again so that a sample could be given to the pharmacological department for further tests."
So, in the spring of 1943, he repeated the synthesis of LSD-25. Quoted below is his entry for this experiment in his laboratory journal of April 19, 1943.
Self-Experiments
17:00: Beginning dizziness, feeling of anxiety, visual distortions, symptoms of paralysis, desire to laugh.
His diary, written later, continues the story:
"Here the notes in my laboratory journal cease. I was able to write the last words only with great effort. By now it was already clear to me that LSD had been the cause of the remarkable experience of the previous Friday, for the altered perceptions were of the same type as before, only much more intense. I had to struggle to speak intelligibly. I asked my laboratory assistant, who was informed of the self-experiment, to escort me home. We went by bicycle, no automobile being available because of wartime restrictions on their use. On the way home, my condition began to assume threatening forms. Everything in my field of vision wavered and was distorted as if seen in a curved mirror. I also had the sensation of being unable to move from the spot. Nevertheless, my assistant later told me that we had traveled very rapidly. Finally, we arrived at home safe and sound, and I was just barely capable of asking my companion to summon our family doctor and request milk from the neighbors.
The dizziness and sensation of fainting became so strong at times that I could no longer hold myself erect, and had to lie down on a sofa. My surroundings had now transformed themselves in more terrifying ways. Everything in the room spun around, and the familiar objects and pieces of furniture assumed grotesque, threatening forms. They were in continuous motion, animated, as if driven by an inner restlessness. The lady next door, whom I scarcely recognized, brought me milk - in the course of the evening I drank more than two liters. She was no longer Mrs. R., but rather a malevolent, insidious witch with a colored mask."
Biological Effects of LSD
The mechanism by which LSD causes such profound affects on the human perception still hasn't been established. What is known, is that as well as the uterine-constricting activity mentioned earlier, LSD stimulates centers of the sympathetic nervous system in the midbrain, which leads to pupillary dilation, increase in body temperature, and rise in the blood-sugar level. LSD also has a serotonin-blocking effect. Serotonin is a hormone-like substance, occurring naturally in various organs of warm-blooded animals. Concentrated in the midbrain, it plays an important role in the propagation of impulses in certain nerves and therefore in the biochemistry of psychic functions. LSD also influences neurophysiological functions that are connected with dopamine, which is another naturally occurring hormone-like substance. Most of the brain centres receptive to dopamine become activated by LSD, while the others are depressed. The structure of LSD is very similar to other hallucinogenic drugs such as mescaline and psilocybin, all of which contain a substituted indole ring (or a related structure).
LSD as an Illegal Drug
Because of its hallucinatory properties, LSD was widely adopted by the hippy culture of the 1960's, who claimed it led to higher states of consciousness and helped them search for religious enlightenment. The Beatles' even wrote a song ('Lucy in the Sky with Diamonds') which describes the psychedelic effects of LSD.
"Picture yourself in a boat on a river with tangerine trees and marmalade sky,
Somebody calls you, you answer quite slowly, a girl with kaleidoscope eyes.
Cellophane flowers of yellow and green towering over your head.
Look for the girl with the sun in her eyes and she's gone.
Lucy in the Sky with diamonds..."
Although LSD is relatively non-toxic and non-addictive, various governments around the world outlawed it after a number of fatal accidents were reported. Such accidents involved, for example, people under the influence of LSD jumping to their deaths off high buildings thinking they could fly. Research in the 60's and 70's showed that there was also a considerable psychological risk with the drug and that high doses, especially in inappropriate settings, often caused panic reactions. For individuals who have a low threshold for psychosis, a bad LSD trip could be the triggering event for the onset of full-blown psychosis. Research on potential therapeutic uses of LSD was abandoned for political reasons in the mid-1970s.
References:
- Organic Chemistry, Morrison and Boyd (Allyn and Bacon, 1983).
- Biochemistry, L. Stryer (W.H. Freeman and Co, San Francisco, 1975).
- Journal of Psychedelic Drugs, Vol. 11 (1-2), 1979.
- LSD - My Problem Child, A. Hoffman, 1978.
EXERCISE: Find the structure and molecular formula for another illegal drug in the United States. Give a brief history (less than 200 words) of the drug and include a reference to information.
Questions? Contact me!