CH 221 Chemical of the Week
Methyl Tertiary-Butyl Ether
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

METHYL TERTIARY-BUTYL ETHER, C5H12O
Methyl tertiary-butyl ether (methyl t-butyl ether, MTBE) is a colorless liquid with a boiling point of 55 °C and a density of 0.74 g/mL. Its molecular structure is below.
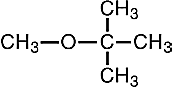
An ether is a compound containing an oxygen atom bonded to two carbon atoms. In MTBE one carbon atom is that of a methyl group, -CH3, and the other is the central atom in a tertiary butyl group, -C(CH3)3. MTBE is made by reacting methanol, made from natural gas, with isobutylene (2-methyl-1-propene) in the liquid state, using an acidic catalyst at 100 °C.
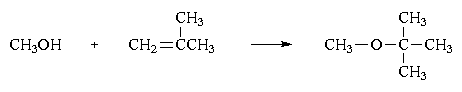
The isobutylene is made from butanes derived from petroleum. Production of MTBE has undergone a tremendous increase, increasing 10 to 20% per year over the past few years. The reason for this rapid increase in production is its use as a gasoline additive.
Gasoline is a mixture of compounds. The majority of these substances are hydrocarbons, compounds that contain only hydrogen and carbon. These substances combust, releasing a great deal of energy, which is harnessed to perform work by the internal combustion engine. When gasoline vapor and air are combined in the cylinder of an engine and the gasoline is ignited, the heat released causes the gases to expand and push the piston down the cylinder. For an engine to operate efficiently, the combustion should occur at the same rate that the piston moves. (In automobile engines, the combustion should occur in about 0.01 sec.) If the combustion is too slow, unburned fuel and incompletely burned fuel will be flushed from the engine as the piston recycles for the next combustion. This wastes the energy content of the fuel and leads to pollution of the air by unburned fuel and carbon monoxide. If the combustion is too fast, faster than the piston can move, damage to the engine will occur. A too rapid explosion produces a knocking sound in the engine and also wastes energy.
Fuels are rated according to their ability to burn smoothly and avoid knocking. This rating is called the octane rating. A measurement is performed by burning the fuel in a special test engine designed for this purpose and observing the amount of knocking the fuel produces. Pure normal heptane, a high-knocking fuel, is assigned an octane number of zero; isooctane (2,2,4-trimethylpentane) is assigned an octane rating of 100. Other fuels are then burned in the test engine, and the knocking is measured. One that knocks as much as a mixture of 90 parts isooctane and 10 parts heptane is assigned an octane rating of 90. Most automobile engines require fuel with octane ratings of 87 to 93 to avoid knocking. (Using a fuel with a lower octane rating than necessary can damage the engine and hinder its performance, but using one with a higher rating than needed does not improve performance.) Because the octane ratings of fuels derived directly from distilled petroleum are too low, additives are used to increase ratings. MTBE is one of these additives. Pure MTBE has an octane rating of 110, and adding it to gasoline increases the octane rating of the fuel. However, the ability of MTBE to increase octane ratings is not the reason for the very great increase in its production over the last decade.
The 1990 amendments to the Clean Air Act spurred the increased production and use of MTBE because of its requirements for the use of oxygenated and reformulated gasoline in 41 cities and metropolitan areas with particular smog and air quality problems. Oxygenated gasoline includes additives whose molecules contain oxygen atoms. Commonly used oxygenating additives are methanol (CH3OH), ethanol (C2H5OH), and MTBE. During winter, when the air is cold, combustion is slow, and gasoline does not burn completely. Incomplete combustion leads to the emission of carbon monoxide and other volatile organic compounds (VOC's) which can cause a variety of health and air quality problems, including increased smog. Introducing additives that are partially oxidized promotes the complete combustion of gasoline, so the engine emits CO2 instead of CO. Reformulated gasoline (RFG) has been refined to remove the more volatile (easily evaporating) components of the gasoline mixture, and includes other additives, including MTBE, that promote cleaner burning. The use of RFG is designed to reduce emissions of VOC's and nitrogen oxides (NOx's), thereby reducing low-level ozone concentrations and smog. EPA regulations concerning RFG went into effect in 1995, and to supply the required oxygenating agents, the production of MTBE has soared. Approximately 30% of the gasoline sold today in the US is reformulated.
Since the inception of the EPA regulations in 1995, significant increases in air quality have been observed nationwide, despite continuously increasing automobile traffic. Smog-causing pollutants, including VOC's, decreased 17% in the four-year period from 1995 to 1999. More stringent regulations went into effect on January 1, 2000, and VOC emissions are expected to drop another 10%, and NOx emissions may drop more than 7%. This would be the equivalent of removing the pollution from 16 million cars nationwide. Additionally, the Northeastern States for Coordinated Air Use Management estimate that the relative cancer risk from gasoline vapors is 19% lower for RFG than for conventional gasoline.
Among the cities required to use RFG are those in southeastern Wisconsin, including Racine, Kenosha, and the Milwaukee metropolitan area. This requirement triggered a controversy over the health effects of MTBE in this area. Some customers of filling stations complained of headaches and other physical maladies, which they attribute to MTBE, though there is no evidence that MTBE is more harmful than other components of gasoline. Some customers also complain of decreased fuel efficiency, but EPA studies have determined that the energy content of RFG is similar to conventional winter gasoline. Conventional gasoline has an energy content of 31350 kJ/L, and RFG is approximately 31150 kJ/L. (The variation in energy content between filling stations is approximately 400 kJ/L.) The lower efficiency of RFG corresponds to a loss of about 0.25 miles per gallon for the average car, which is similar to driving into a 20 mile-per-hour headwind, and is only 10% of the loss associated with driving with air conditioning in operation.
The most important concern about MTBE has been its detection in surface and groundwater sources, particularly since the inception of the EPA's rules in 1995. MTBE can enter the groundwater through leaking gasoline tanks and lines, and through improper disposal or dumping of gasoline. However, MTBE is much more soluble in water than most other components of gasoline, so its transport by groundwater is much faster than that of the other components. (Ironically, MTBE in gasoline often allows detection of a leaking tank years before it would be possible if the tank held conventional gasoline.) Even at extremely low levels, MTBE is distasteful and has a foul odor. Currently, only the state of California requires routine monitoring of drinking-water sources for MTBE, and it has set a secondary standard of only 5 parts per billion (ppb). (Secondary standards are set on the basis of aesthetics such as taste and smell, unlike primary standards, which are determined on the basis of toxic risk.) In June 1999, only 3.7% of California drinking water samples had detectable levels of MTBE, and many of those were still below the 5 ppb secondary standard. In Wisconsin, MTBE has been detected in groundwater at nine spill sites, at levels ranging from 0.47 to 1.1 ppb. These levels are well below the EPA advisory standard of 20 to 40 ppb, which is set at a level 20,000 to 100,000 times lower than the range of exposure levels at which cancer or toxic effects have been observed in rodents. In April of 1999, the State of California adopted a ban on MTBE in gasoline, to be phased in over a 4-year period. In September of 1999, the EPA recommended to Congress that MTBE be phased out as a gasoline additive, provided it could be accomplished without affecting the RFG pollutant guidelines. Therefore, it is likely that ethanol will soon largely replace MTBE as an oxygenating additive.
References
- "Reformulated Gasoline: Five Years Later" by Dennis Koepke, Wisconsin Department of Natural Resources fact sheet, October 1999.
- "Emission Facts: Reformulated Gasoline" United States Environmental Protection Agency (document EPA-420-F-99-040), November 1999.
- "Phase II Reformulated Gasoline: the next step toward cleaner air" USEPA (document EPA420-F-99-042), November 1999.
- From congressional testimony of M.T. Oge, Director, Office of Mobile Sources, USEPA, Sept. 14, 1999
- "Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Methyl Tertiary-Butyl Ether (MtBE)" United States Environmental Protection Agency (document EPA-822-F-97-009), December 1997.
- "Not to Worry. Gasoline additive has (probably) not fouled water here." Bill Leuders, Isthmus, Jan. 28, 2000, v 25, no 4, p 7.
EXERCISE: Using the internet or the library, find an example of a gasoline alternative fuel source not listed in this article and write a short (less than 100 words) summary of its benefits and drawbacks when compared with gasoline. Include any references you utilize.
Questions? Contact me!