CH 223 Chemical of the Week
Liquid Crystals
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

LIQUID CRYSTALS
To those who know that substances can exist in three states, solid, liquid, and gas, the term "liquid crystal" may be puzzling. How can a liquid be crystalline? However, "liquid crystal" is an accurate description of both the observed state transitions of many substances and the arrangement of molecules in some states of these substances.
Many substances can exist in more than one state. For example, water can exist as a solid (ice), liquid, or gas (water vapor). The state of water depends on its temperature. Below 0°C, water is a solid. As the temperature rises above 0 °C, ice melts to liquid water. When the temperature rises above 100°C, liquid water vaporizes completely. Some substances can exist in states other than solid, liquid, and vapor. For example, cholesterol myristate (a derivative of cholesterol) is a crystalline solid below 71 °C. When the solid is warmed to 71 °C, it turns into a cloudy liquid. When the cloudy liquid is heated to 86 °C, it becomes a clear liquid. Cholesterol myristate changes from the solid state to an intermediate state (cloudy liquid) at 71 °C, and from the intermediate state to the liquid state at 86 °C. Because the intermediate state exits between the crystalline solid state and the liquid state, it has been called the liquid crystal state.
 |
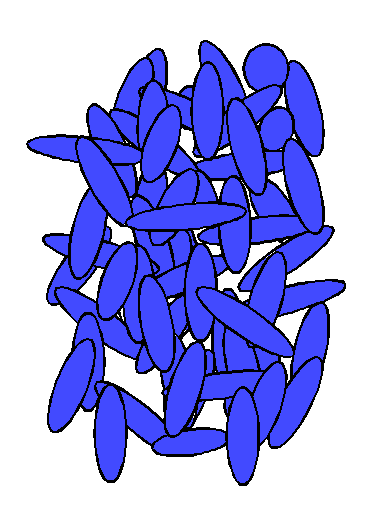 |
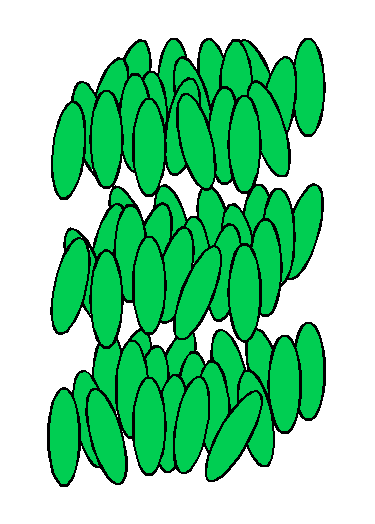 |
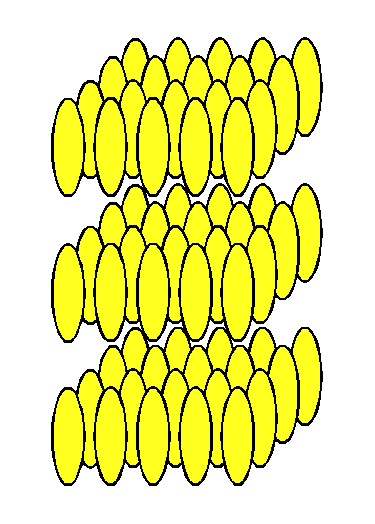
| Figure 1. Arrangement of molecules in a solid crystal. | Figure 2. Arrangement of molecules in a liquid. | Figure 3. Arrangement of molecules in a liquid crystal. |
"Liquid crystal" also accurately describes the arrangement of molecules in this state. In the crystalline solid state, as represented in Figure 1, the arrangement of molecules is regular, with a regularly repeating pattern in all directions. (Molecules of substances with a liquid crystal state are generally oblong and rigid, that is, rod-shaped.) The molecules are held in fixed positions by intermolecular forces. As the temperature of a substance increases, its molecules vibrate more vigorously. Eventually, these vibrations overcome the forces that hold the molecules in place, and the molecules start to move. In the liquid state, this motion overcomes the intermolecular forces that maintain a crystalline state, and the molecules move into random positions, without pattern in location or orientation, as represented in Figure 2.
In materials that form liquid crystals, the intermolecular forces in the crystalline solid are not the same in all directions; in some directions the forces are weaker than in other directions. As such a material is heated, the increased molecular motion overcomes the weaker forces first, but its molecules remain bound by the stronger forces. This produces a molecular arrangement that is random in some directions and regular in others. The arrangement of molecules in one type of liquid crystal is represented in Figure 3. The molecules are still in layers, but within each layer, they are arranged in random positions, although they remain more or less parallel to each other. Within layers, the molecules can slide around each other, and the layers can slide over one another. This molecular mobility produces the fluidity characteristic of a liquid.
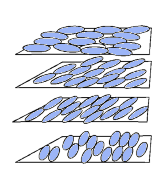 Figure 4. Arrangement of molecules in a twisted nematic liquid crystal. |
There are other molecular arrangements in liquid crystals. Many liquid crystals of technological significance have the arrangement represented in Figure 4. Liquid crystals with this arrangement are called twisted nematic liquid crystals. In this arrangement, the layers contain the long axes of the molecules. Furthermore, the long axes rotate by a small angle from one layer to the next. Twisted nematic liquid crystals are used in temperature-sensing devices that change color. They are also the most common type used in the liquid crystal displays (LCDs) found in calculators and watches. Both of these applications depend on the way liquid crystals interact with light.
When light strikes a twisted nematic liquid crystal, some of the light is reflected. Only light with a wavelength equal to the spacing between layers of similar orientation is reflected. Therefore, the reflected light will appear colored. As the temperature of the liquid crystal changes, the spacing between layers also changes. The change in spacing changes the wavelength of the reflected light and its observed color. Therefore, the color of the reflected light is an indication of the temperature of the liquid crystal. Different substances form twisted nematic liquid crystals over different temperature ranges, and so a wide temperature range can be covered by using several substances.
When polarized light passes through a twisted nematic liquid crystal, the plane of polarization rotates. The degree of rotation depends on the number of layers of molecules the light encounters in the liquid crystal. If the axes of the molecules in the layer from which the light exits are at an angle of 90 degrees to those in the layer of entry, then the plane of polarization of the light rotates by 90 degrees. The ability of twisted nematic liquid crystals to rotate the plane of polarized light is exploited in LCDs. Figure 5 is a schematic diagram of an LCD. Ambient light enters from the right and is polarized by a polarizing filter. The polarized light passes through the front glass wall of the display, then through a transparent, electrically conductive coating on the glass, and into the liquid crystal. The thickness of the liquid crystal is sufficient to rotate the plane of the polarized light by 90 degrees. At the back of the display, the light passes through another electrically conductive coating, glass plate, and polarizing filter. This rear polarizing filter is placed with its axis at 90 degrees to the front filter. The polarized light passes through this rear filter, because its polarization was also rotated by 90 degrees by the liquid crystal. At the back of the LCD is a mirror that reflects the light back through the cell. The light retraces its path, and an observer sees the display as relatively bright. When an electric potential is applied between the two conductive coatings, the resulting electric field affects the positions of molecules in the liquid crystal. The molecules tend to turn so they align with the electric field. When this happens, the plane of polarized light passing through the cell is no longer rotated by 90 degrees, and it cannot pass through the rear filter. Therefore, it is no longer reflected back to an observer, and the area appears dark. By the selective charging of areas of the conductive coating, patterns of dark digits or letters against a bright background are formed.
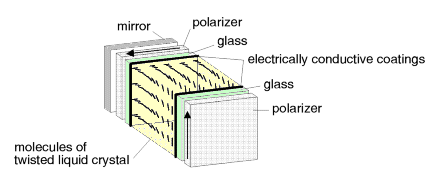 Figure 5. Schematic diagram of liquid crystal display. |
Substances that form liquid crystal structures are quite common. Approximately 0.5% of known carbon compounds have liquid crystal states. These structures are especially common in living organisms, where cell walls are composed of molecules in a liquid crystal arrangement of parallel molecules in layers. A more mundane instance of liquid crystals is the opalescent fluid that forms in the bottom of a soap dish. Soap molecules have the appropriate oblong shape and, when mixed with a little water, assume a liquid crystal arrangement.
EXERCISE: Search the internet for liquid crystal information. What internet site appeals to you the most and why? Be sure to include the URL for the internet site.
Questions? Contact me!