CH 223 Chemical of the Week
Chelating Agents
Explore a new chemical in depth every week! Students can receive an extra credit point by answering the question(s) posted in the "Chemical of the Week" article and submitting the answer to the instructor by 9 AM on the due date listed. Questions should be addressed to the instructor.
Submit your answers to Chemical of the Week questions by turning a printed copy of your work to the instructor directly (in lecture or in his mailbox) or by emailing me directly.

CHELATING AGENTS
A chelating agent is a substance whose molecules can form several coordinate bonds to a single metal ion. That is, a chelating agent is a polydentate ligand. The most common and most widely used chelating agents are those that coordinate to metal ions through oxygen or nitrogen donor atoms, or through both. Chelating agents that coordinate through sulfur in the form of -SH (thiol or mercapto) groups are not as common in commercial applications, but they perform a significant role in complexing metal ions in biological systems. Three widely used chelating agents are ethylenediamine, ethylenediaminetetraacetic acid, and dimercaprol.
ETHYLENEDIAMINE

Ethylenediamine (molar mass 60.10 g) is a colorless, clear liquid with a boiling point of 116°C and a melting point of about 8 °C. It has an ammonia-like odor, and it dissolves in water to form an alkaline solution. Ethylenediamine is prepared commercially by heating 1,2-dichloroethane with aqueous ammonia under pressure at 100-180 °C.
Cl-CH2-CH2-Cl + 2 NH3 ![]() H2N-CH2-CH2-NH2 + 2 HCl
H2N-CH2-CH2-NH2 + 2 HCl
Ethylenediamine is one of several products of the reaction, and it is separated from the others by distillation.
Ethylenediamine reacts with a variety of transition metal ions to form complex ions. Each complex ion has its own characteristic properties. (Refer to the laboratory experiment with nickel complexes.)
ETHYLENEDIAMINETETRAACETIC ACID (EDTA)

EDTA (molar mass 292.3 g) is a colorless crystalline material which decomposes when heated to above 240 °C. It is only slightly soluble in water. However, its sodium salts are quite soluble in water. The commercial preparation involves the reaction of ethylenediamine, formaldehyde (HCHO), and sodium cyanide.
H2N-CH2-CH2-NH2 + HCHO + NaCN ![]() (Na+-1OCOCH2)2N-CH2-CH2-N(CH2COO-1Na+)2
(Na+-1OCOCH2)2N-CH2-CH2-N(CH2COO-1Na+)2
EDTA is also prepared commercially by the reaction of ethylenediamine and chloroacetic acid.
H2N-CH2-CH2-NH2 + ClCH2COOH ![]() (HOOCCH2)2N-CH2-CH2-N(CH2COOH)2
(HOOCCH2)2N-CH2-CH2-N(CH2COOH)2
Neither of these equations shows the stoichiometry of the reactions, but each identifies the major chemical species involved.
EDTA can be a tetradentate or a hexadentate ligand. In a calcium compound with the formula K2[Ca(EDTA)], EDTA is a tetradentate ligand. In this compound, chelation involves the two nitrogen atoms and two oxygen atoms in separate carboxyl (-COO-1) groups. EDTA is a sequestering (metal-complexing) agent. It is used extensively as a stabilizing agent in the food industry. This compound, as the disodium or calcium disodium salt, promotes color retention in dried bananas, beans, chick peas, canned clams, pecan pie filling, frozen potatoes, and canned shrimp. It improves flavor retention in canned carbonated beverages, salad dressings, mayonnaise, margarine, and sauces. It retards struvite formation in canned crab meat and shrimp. It inhibits rancidity in salad dressings, mayonnaise, sauces, and sandwich spreads. EDTA salts are used in foods at levels ranging from 33 to 800 ppm.
Ethylenediaminetetraacetic acid sequesters metal ions, binding them so they cannot react in undesirable ways. EDTA deactivates metallo-enzymes by removing the metal ion from the enzyme. In food, some of these enzymes catalyze the reactions that produce spoilage. EDTA dissolves the CaCO3 scale deposited from hard water without the use of corrosive acid. EDTA is also used as an anticoagulant for stored blood in blood banks. It prevents coagulation by sequestering the calcium ions required for clotting. As an antidote for lead poisoning, calcium disodium EDTA exchanges its chelated calcium for lead, and the resulting lead chelate is rapidly excreted in the urine. The calcium salt of EDTA, administered intravenously, is also used in the treatment of acute cadmium and iron poisoning.
DIMERCAPROL
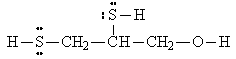
Dimercaprol (2,3-dimercapto-1-propanol) is a water-insoluble compound with an offensive odor. Compounds that contain the -SH group are known as mercaptans and are responsible for the strong odors associated with certain animals, such as skunks and ferrets. This chemical was originally employed to treat the toxic effects of an arsenic-containing mustard gas called Lewisite [dichloro(2-chlorovinyl)arsine], which was used in World War I.
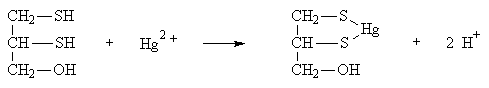
Dimercaprol is the most widely used antidote for arsenic, mercury, antimony, and gold poisoning. The chelated metal cannot enter living cells and is rapidly excreted from the body. Since dimercaprol is water insoluble, it is dissolved in an oil base (often peanut oil) and injected intramuscularly.
EXERCISE: Find a chelating agent not listed here and write its chemical formula and structure. Find an example of when the chelate is used in nature or science. List the source by which you found the chelate.
Questions? Contact me!