Multiple
Choice
Identify the letter of the choice that best completes the statement or answers the
question.
|
|
|
1.
|
Which of the following
equations is the solubility product for magnesium iodate,
Mg(IO3)2? a. | Ksp =
[Mg2+][I-]2[O2-]6 | b. | Ksp =
[Mg2+][I-]2[3O2-]2 | c. | Ksp = [Mg2+]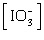 | d. | Ksp = [Mg2+]2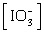 | e. | Ksp = [Mg2+]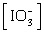 2 2 | | |
|
|
|
2.
|
The solubility of
PbCl2 in water is 4.4 g/L at 25 °C. What
is the value of Ksp for PbCl2? a. | 4.0 × 10-6 | b. | 1.6 × 10-5 | c. | 2.5 × 10-4 | d. | 5.0 × 10-4 | e. | 3.4 × 102 | | |
|
|
|
3.
|
The Ksp of
PbBr2 is 6.6 × 10-6 at 25 °C. What is the concentration of Br- in a saturated solution of
PbBr2(aq)? a. | 2.6 ×
10-3 M | b. | 1.2 ×
10-2 M | c. | 1.9 ×
10-2 M | d. | 2.4 ×
10-2 M | e. | 3.8 ×
10-2 M | | |
|
|
|
4.
|
The Ksp of
AgBr is 5.4 × 10-13 at 25 °C. Calculate the molar solubility of AgBr in 0.015 M NaBr(aq) at 25 °C. a. | 8.1 ×
10-15 mol/L | b. | 3.6 ×
10-11 mol/L | c. | 1.1 ×
10-8 mol/L | d. | 7.3 ×
10-7 mol/L | e. | 4.9 ×
10-5 mol/L | | |
|
|
|
5.
|
At 25 °C, only 1.04 mg Cu(NO3)2 will dissolve per liter of a solution
that is buffered at pH 6.80. What is the value of Ksp for Cu(OH)2? The
molar mass of Cu(NO3)2 is 187.6 g/mol. a. | 2.2 ×
10-20 | b. | 1.4 ×
10-19 | c. | 4.1 ×
10-18 | d. | 3.5 ×
10-13 | e. | 8.8 ×
10-13 | | |
|
|
|
6.
|
What is the molar solubility of
Fe(OH)3(s) in a solution that is buffered at pH 2.75 at 25 °C? The Ksp of Fe(OH)3 is 6.3 × 10-38 at 25 °C. a. | 1.1 ×
10-29 mol/L | b. | 1.1 ×
10-26 mol/L | c. | 2.0 ×
10-15 mol/L | d. | 2.2 ×
10-10 mol/L | e. | 3.5 ×
10-4 mol/L | | |
|
|
|
7.
|
At what pH will an aqueous
solution of 0.210 M Mn2+ begin to precipitate as Mn(OH)2 at 25 °C? The Ksp for Mn(OH)2 is 1.9 × 10-13 at 25 °C. a. | 3.94 | b. | 6.02 | c. | 7.98 | d. | 9.86 | e. | 10.06 | | |
|
|
|
8.
|
An aqueous solution contains
0.010 M Br- and 0.010 M I-. If Ag+ is added until AgBr(s) just
begins to precipitate, what are the concentrations of Ag+ and I-?
(Ksp of AgBr = 5.4 ×
10-13, Ksp of AgI = 8.5 ×
10-17) a. | [Ag+] = 5.4 ×
10-11 M, [I-] = 1.0 ×
10-2 M | b. | [Ag+] = 8.5 × 10-15 M, [I-] = 1.0 ×
10-2 M | c. | [Ag+] = 5.4 × 10-11 M, [I-] = 1.6 ×
10-6 M | d. | [Ag+] = 8.5 × 10-15 M, [I-] = 6.4 ×
101 M | e. | [Ag+] = 8.5 × 10-15 M, [I-] = 1.6 ×
10-6 M | | |
|
|
|
9.
|
The following anions can be
separated by precipitation as silver salts: Cl-, Br-, I-,
CrO42-. If Ag+ is added to a solution containing the four anions,
each at a concentration of 0.10 M, in what order will they precipitate?
Compound | K sp | AgCl | 1.8 ×
10 -10 | Ag2CrO4 | 1.1 ×
10-12 | AgBr | 5.4 ×
10 -13 | AgI | 8.5 ×
10 -17 | | |
a. | AgCl →
Ag2CrO4 → AgBr →
AgI | b. | AgI → AgBr
→ Ag2CrO4 →
AgCl | c. | Ag2CrO4 → AgCl
→ AgBr →
AgI | d. | Ag2CrO4 → AgI
→ AgBr →
AgCl | e. | AgI → AgBr
→ AgCl →
Ag2CrO4 | | |
|
|
|
10.
|
Given the following
reactions,
AgCl(s) 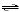 Ag+(aq) + Cl-(aq) Ksp = 1.8
× 10-10 Ag+(aq) + Cl-(aq) Ksp = 1.8
× 10-10
Ag+(aq) + 2 NH3(aq)
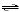 Ag(NH3)2+(aq) Kform = 1.6 × 107 Ag(NH3)2+(aq) Kform = 1.6 × 107
determine the equilibrium constant for the reaction below.
AgCl(s) + 2 NH3(aq) 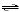 Ag(NH3)2+(aq) + Cl-(aq)
Ag(NH3)2+(aq) + Cl-(aq)
a. | 1.1 ×
10-17 | b. | 2.9 ×
10-3 | c. | 3.5 ×
102 | d. | 1.6 ×
107 | e. | 8.9 ×
1016 | | |
|