Multiple
Choice
Identify the letter of the choice that best completes the statement or answers the
question.
|
|
|
1.
|
Given a solution of 0.10 M
NH3(aq), what is the effect of adding NH4Cl(s) to this
solution?
1. | The pH will decrease. | 2. | The concentration of NH3 will increase. | 3. | The concentration of H3O+ will increase. | | |
a. | 1 only | b. | 2 only | c. | 3 only | d. | 1 and 3 | e. | 1, 2, and 3 | | |
|
|
|
2.
|
What is the pH of an aqueous
solution of 0.30 M HF and 0.15 M F-? (Ka of HF = 7.2 × 10-4) a. | 1.83 | b. | 2.84 | c. | 3.14 | d. | 3.44 | e. | 10.86 | | |
|
|
|
3.
|
Which of the following
combinations would be best to buffer an aqueous solution at a pH of 2.0? a. | H3PO4 and H2PO4-,
Ka1 = 7.5 × 10-3 | b. | HNO2 and NO2-, Ka = 4.5
× 10-4 | c. | CH3CO2H and CH3COO-, Ka
= 1.8 × 10-5 | d. | H2PO4- and HPO42-,
Ka2 = 6.2 × 10-8 | e. | NH4+ and NH3, Ka = 5.7 × 10-10 | | |
|
|
|
4.
|
What is the pH of the buffer
that results when 4.0 g of NH3 and 8.0 g of NH4Cl are diluted with water to a
volume of 0.50 L? (Ka of NH4+ = 5.6 × 10-10) a. | 8.95 | b. | 9.06 | c. | 9.25 | d. | 9.45 | e. | 9.55 | | |
|
|
|
5.
|
What is the pH of a buffer that
results when 33 g NaHCO3 is mixed with 100.0 mL of 2.50 M NaOH and diluted with water to
250 mL? (Ka for HCO3- = 4.8 × 10-11) a. | 8.20 | b. | 10.07 | c. | 10.12 | d. | 10.32 | e. | 10.56 | | |
|
|
|
6.
|
A buffer is prepared by
combining 250 mL of 0.25 M NaOH and 250 mL of a 0.600 M weak acid, HA. If the pH of the buffer is
6.33, what is the pKa of the acid? a. | 5.95 | b. | 6.18 | c. | 6.33 | d. | 6.48 | e. | 6.71 | | |
|
|
|
7.
|
How many moles of HCl must be
added to 1.00 L of 0.72 M NH3 to make a buffer with a pH of 9.50? (Ka of
NH4+ = 5.6 ×
10-10) a. | 0.26 mol | b. | 0.31 mol | c. | 0.41 mol | d. | 0.46 mol | e. | 1.3 mol | | |
|
|
|
8.
|
A volume of 25.0 mL of 0.100 M
HCO2H(aq) is titrated with 0.100 M NaOH(aq). What is the pH after the addition of 12.5 mL
of NaOH? (Ka for HCO2H = 1.8 ×
10-4) a. | 2.52 | b. | 3.74 | c. | 4.74 | d. | 7.00 | e. | 10.26 | | |
|
|
|
9.
|
A 25.0 mL sample of 0.0200 M
NH3(aq) is titrated with 0.0100 M HCl(aq). What is the pH at the equivalence point?
(Kb of NH3 = 1.8 ×
10-5) a. | 3.46 | b. | 5.48 | c. | 5.72 | d. | 8.25 | e. | 10.54 | | |
|
|
|
10.
|
Potassium hydrogen phthalate
(molar mass = 204.2 g/mol) is used to standardize sodium hydroxide. If 26.37 mL of NaOH(aq) is
required to titrate 0.7719 g KHP to the equivalence point, what is the concentration of the
NaOH(aq)?
HC8H4O4-(aq) + OH-(aq) 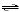 C8H4O42-(aq) + H2O(l)
C8H4O42-(aq) + H2O(l)
a. | 0.02036 M | b. | 0.02937 M | c. | 0.09968 M | d. | 0.1433 M | e. | 5.977 M | | |
|