Multiple
Choice
Identify the letter of the choice that best completes the statement or answers the
question.
|
|
|
1.
|
According to the
Brønsted-Lowry definition, an acid a. | increases the H3O+
concentration in an aqueous solution. | b. | increases the OH- concentration
in an aqueous solution. | c. | is a proton
acceptor. | d. | is a proton donor. | e. | is an electron pair-acceptor. | | |
|
|
|
2.
|
According to the
Brønsted-Lowry definition, a base a. | increases the H3O+
concentration in an aqueous solution. | b. | increases the OH- concentration
in an aqueous solution. | c. | is a proton
acceptor. | d. | is a proton donor. | e. | is an electron-pair donor. | | |
|
|
|
3.
|
In the following
reaction
HF(aq) + HPO42-(aq) 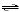 F-(aq) +
H2PO4-(aq) F-(aq) +
H2PO4-(aq)
a. | HF is an acid and F- is its
conjugate base. | b. | HF is an acid and
HPO42- is its conjugate base. | c. | HPO42- is an acid and H2PO4- is
its conjugate base. | d. | H2PO4- is
an acid and F- is its conjugate base. | e. | HPO42- is an acid and HF is its conjugate
base. | | |
|
|
|
4.
|
Which acid is a potentially
dangerous component of rhubarb leaves? a. | oxalic | b. | arsenous | c. | arsenic | d. | nitric | e. | selenic | | |
|
|
|
5.
|
Which of the following
molecules or ions is amphiprotic in water? a. | NH4+ | b. | HF | c. | CH3CO2H | d. | CN- | e. | HCO3- | | |
|
|
|
6.
|
What is the pH of 0.28 M
HNO3(aq) at 25 °C? (Kw = 1.0
× 10-14) a. | -0.55 | b. | 0.28 | c. | 0.55 | d. | 1.27 | e. | 13.45 | | |
|
|
|
7.
|
What is the
H3O+ concentration of an aqueous solution with a pH of 12.17? a. | 6.8 × 10-13
M | b. | 1.9 ×
10-9 M | c. | 5.2 ×
10-6 M | d. | 1.5 ×
10-2 M | e. | 1.1 M | | |
|
|
|
8.
|
A solution is made by diluting
0.50 mol NaClO to a volume of 3.0 L with water. What is the pH of the solution? (Kb
of ClO- = 2.9 × 10-7) a. | 3.66 | b. | 7.46 | c. | 10.34 | d. | 10.58 | e. | 13.22 | | |
|
|
|
9.
|
Which of the following chemical
equations corresponds to Ka3 for phosphoric acid? a. | HPO42-(aq) + H2O(l)
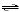 PO43-(aq) + H3O+(aq) PO43-(aq) + H3O+(aq) | b. | PO43-(aq) + H2O(l)
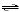 HPO42-(aq) + OH-(aq) HPO42-(aq) + OH-(aq) | c. | H3PO4(aq) + H2O(l)
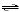 H2PO4-(aq) +
H3O+(aq) H2PO4-(aq) +
H3O+(aq) | d. | H3PO4(aq) + 3
H2O(l) 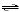 PO43-(aq)
+ 3 H3O+(aq) PO43-(aq)
+ 3 H3O+(aq) | e. | H2PO4-(aq) + H2O(l) 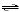 HPO42-(aq) +
H3O+(aq) HPO42-(aq) +
H3O+(aq) | | |
|
|
|
10.
|
All of the following compounds
are acids containing chlorine. Which compound is the weakest acid? a. | HCl | b. | HClO | c. | HClO2 | d. | HClO3 | e. | HClO4 | | |
|