Multiple
Choice
Identify the letter of the choice that best completes the statement or answers the
question.
|
|
|
1.
|
All of the following statements
concerning nuclei are true EXCEPT a. | only hydrogen-1 and helium-3 have more
protons than neutrons. | b. | from He to Ca, stable nuclei have roughly
equal numbers of protons and neutrons. | c. | most stable isotopes have odd numbers of
both protons and neutrons. | d. | the neutron to proton ratio in stable
nuclei increases as mass increases. | e. | beyond calcium, the neutron to proton
ratio is always greater than one. | | |
|
|
|
2.
|
The molar nuclear mass of
carbon-14 is 14.003241 g/mol. The molar mass of a proton is 1.007825 g/mol. The molar mass of a
neutron is 1.008665 g/mol. Calculate the binding energy of C-14. (c = 3.00 × 108 m/s) a. | 1.13 ×
10-1 J/mol | b. | 1.13 ×
10-4 J/mol | c. | 1.02 ×
1013 J/mol | d. | 1.00 ×
1016 J/mol | e. | 1.00 ×
1019 J/mol | | |
|
|
|
3.
|
Strontium-90 has a half-life of
28.1 years. Starting with 2.08 mg of this isotope, how much would remain after 112.4
years? a. | 0.13 mg | b. | 0.26 mg | c. | 0.52 mg | d. | 1.04 mg | e. | 1.56 mg | | |
|
|
|
4.
|
What is the half-life of an
isotope if the decay constant is 7.2 ×
10-4 s-1? a. | 1.0 ×
10-3 s | b. | 1.4 ×
10-3 s | c. | 2.4 ×
10-3 s | d. | 4.2 ×
102 s | e. | 9.6 ×
102 s | | |
|
|
|
5.
|
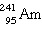 is used in
many home smoke alarms. If 25% of the americium in a smoke detector decays in 180 years, what is the
half-life of this isotope? is used in
many home smoke alarms. If 25% of the americium in a smoke detector decays in 180 years, what is the
half-life of this isotope?a. | 1.9 ×
102 yr | b. | 2.1 ×
102 yr | c. | 3.6 ×
102 yr | d. | 4.3 ×
102 yr | e. | 1.0 ×
103 yr | | |
|
|
|
6.
|
What role do the cadmium
control rods play in a fission reactor? a. | The rods control the rate of fission by
absorbing neutrons. | b. | The cadmium combines with spent uranium
fuel to produce a non-radioactive product. | c. | The rods focus the neutrons toward the
center of the reactor. | d. | The cadmium acts as a catalyst, enabling
fission to occur at lower temperatures. | e. | The rods move forward and backward,
driving the pistons that turn the turbines. | | |
|
|
|
7.
|
Which of the following elements
undergoes nuclear fusion to provide the primary source of energy from the sun? a. | helium | b. | uranium | c. | hydrogen | d. | carbon | e. | nitrogen | | |
|
|
|
8.
|
What do scientists call the
sequence of rapidly occurring reactions that results when a nuclear fission reaction produces enough
neutrons to produce more fission reactions? a. | nucleation | b. | chain reaction | c. | nuclear fusion | d. | neutron multi-emission | e. | binding energy | | |
|
|
|
9.
|
Complete the following fusion
reaction.
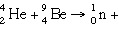 _____ _____
a. | | b. | 2 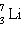 | c. | | d. | | e. | | | |
|
|
|
10.
|
What percentage of the world's
electricity is supplied by nuclear fusion reactors?
|