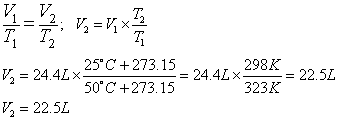Practice Problems Week 8
1. What is the volume of a gas sample at 755 mmHg if it occupies 6.75 L at 875 mmHg? (Assume constant T & n)
OK. First we decide what form of the General Gas Law (GGL) or Ideal Gas Law (IGL) we use. We are not given a number of moles, a density or a number of molecules, so we don’t use the IGL. It looks like we have a pressure and volume and a second pressure. So, it’s a PV problem. So, it’s P1V1 = P2V2 which comes from Boyle’s Law. We’re given P1 = 875 mmHg and V1 = 6.75 L. We could have chosen the subscripts 2 if we wanted to). We’re also given P2. The question asks for V2. So let’s take Boyle’s Law and solve for V2:
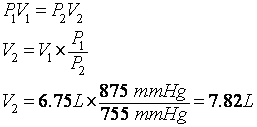
2. What is the temperature in °C of 5.75 m3 of a gas if at 2.68 m3 it has a temperature of 27°C?
It looks like this
problem deals with temperature and volume. So we use the form of the gas law
with those variables: 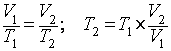 . The question asks
for the final temperature (T2), so let’s solve for that and then
substitute all of the given stuff into it:
. The question asks
for the final temperature (T2), so let’s solve for that and then
substitute all of the given stuff into it:
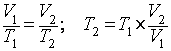
But we ALWAYS remember that we have to use temperature in KELVINS when we use gas equations. So we gotta convert the temperatures first: T1 = 27ºC + 273.15 = 300K. Remember the rules for addition and subtraction regarding decimal places. So now we can substitute in our equation:
![]()
3. What is the volume of a sample of gas at 25°C if it occupies 24.4 L at 50°C? (Assume constant P & n)
This looks like another volume – temperature problem. But
this time we have to calculate a volume. So let’s take our general V-T
equation: 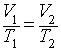 and solve it for a
temperature. It looks like the final temperature, T2, is 25ºC, so T1 = 50ºC and V1 is 24.4 L. So let’s solve for V2 and
substitute values:
and solve it for a
temperature. It looks like the final temperature, T2, is 25ºC, so T1 = 50ºC and V1 is 24.4 L. So let’s solve for V2 and
substitute values: