Practice Problems Week 6
1. How many atoms of aluminum are in 6.75 moles of Al?
![]()
2. How many moles are in ![]() grams of iodine atoms?
grams of iodine atoms?
![]()
3. How many moles are in ![]() grams of iodine molecules?
grams of iodine molecules?
![]()
4. What is the mass, in grams, of one mole of silver?
![]()
5. What is the mass, in grams, of one atom of silver?
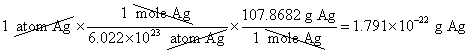
6. How many atoms of lithium are in 25.0g of lithium?
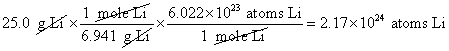
7. How many grams of gold are in ![]() atoms of gold?
atoms of gold?
![]()
8. How many molecules of N2O3 are
in 5.55 picograms of N2O3
?
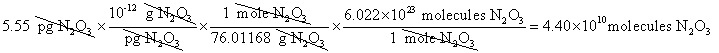
9. How many nitrogen atoms are in 1.75 grams of Al(NO3)3 ?
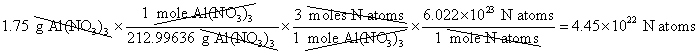
10. How many grams of Al(NO3)3 are required to produce ![]() atoms of oxygen?
atoms of oxygen?
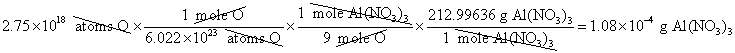
11. For each of the following, (a) write a balanced
chemical equation; (b) indicate the type of reaction (combination,
decomposition, single replacement (displacement), or double replacement (ion
exchange)?
(i)
Ammonium
phosphate + nickel(II) nitrate -> nickel(II) phosphate + Ammonium nitrate
(a)
![]()
(b)
Double displacement
(replacement, partner exchange, ion exchange are synonyms)
(ii)
Aluminum +
sulfuric acid (hydrogen sulfate)
(a)
![]() ( (g) and up arrow
both indicate gas evolved)
( (g) and up arrow
both indicate gas evolved)
(b)
Single
replacement
(iii)
Aluminum +
bromine ->
(a)
![]()
(b)
Combination
(iv)
Potassium
nitrate -> potassium nitrite + oxygen
(a)
![]()
(b)
Decomposition